Article Summary 26/01/24
Article title
Antibody-lectin chimeras for glyco-immune checkpoint blockade
Nature Biotechnology
Tags
Glycan; Checkpoint blockade therapy; Engineered antibody
Introduction
Checkpoint blockade therapy has been used to treat nearly 50% cancer patients in US, but suffered from low response (20% patient) and drug resistance. New checkpoint blockade strategy is needed. Glycans is an attractive target for checkpoint blockade. On cancer cell surface, glycans such as sialic acid regulate immune cell functions, helping immune escape of cancer cell. Targeting those glycans is a checkpoint blockade therapy. Currently, lectin (protein binding glycans) binders towards glycans have low affinity with cancer cells, limiting their application on this therapy. A glycan checkpoint-targeted therapy is urgently needed.
This work
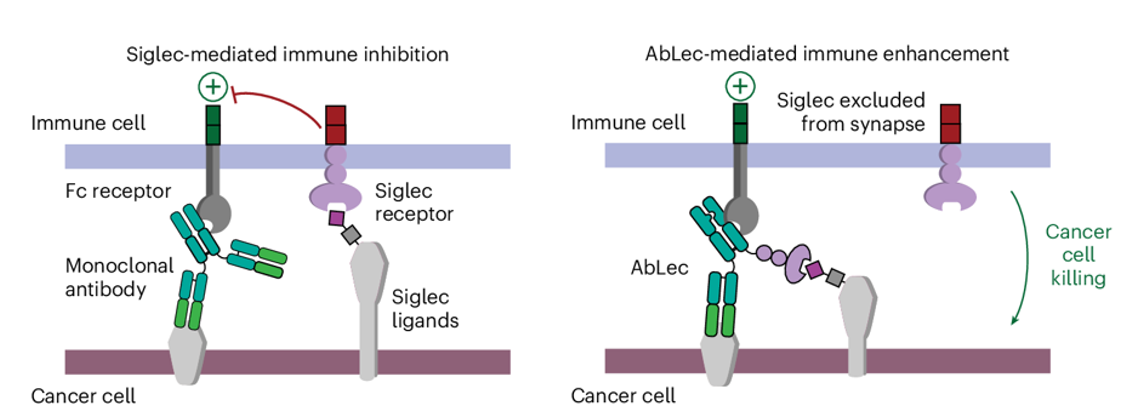
Carolyn Bertozzi et al. developed AbLec, in which cancer cell-related antibody (such as HER2), was fused with lectin to form a chimera. They demonstrated AbLec could enhance immune function in cells and inhibit cancer cell growth in mice. AbLec exerts its function through eliminating the Siglec Ab (the intrinsic lectin receptor) enrichment in immune synapse, thus block the immune escape.
Next, they expanded the range of AbLec to different cancer cell antigens and different lectin receptor, demonstrating its universality across most cancers. Also, fusing lectin receptor with another checkpoint blockage antibody, such as PD-1 and CD47, showed effect in cells.
In summay, the AbLec strategy provided a path to block glycan immunologic checkpoint and established a wide platform to expand the use of AbLex chimera.
doi
10.1038/s41587-025-02884-6