Article Summary 7/19/25
Article title
Serine ADPr on histones and PARP1 is a cellular target of ester-linked ubiquitylation
Journal
Nature Chemical Biology
Tags
PARP1; ADPr-ubiquitination; Mass spectrometry; PTMs proteomics
Introduction
Crosstalks between post-translational modifications have been widely explored. Notably, for ADP-ribosylation involved in DNA repair and ubiquitination pivoted in protein degradation and signal transduction, complex interplays are established such as Ub42R ADP-ribosylation (found in bacteria Legionella) and ADP-ribose ubiquitination. ADP-ribose ubiquitination is introduced by DELTEX E3 ligase in vitro but no evidence of this composite modification is found in cell. It is attributed to the lack of mass spectrometry methodologies to probe the ADPr-Ub modification in high sensitivity.
This work
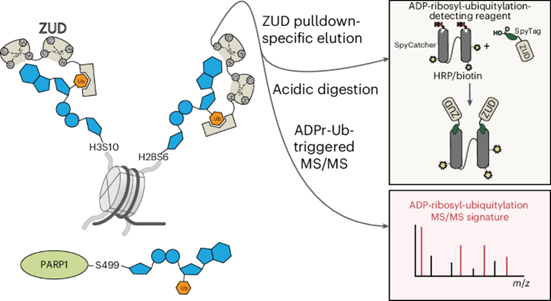
The article first demonstrated the inability of current sample procedure to detect ADPr-Ub modification. Then they established a strategy to probe cellular ADPr-Ub sites. By using ZUD domain of RNF114, which could bind ADP-Ub modification, they optimized the mass spectrometry sample preparation procedure and detect ADPr-Ub sites.
In general, ADPr-Ub modified proteins are enriched in ZUD beads, and no basic condition in sample preparation due to lability of oxyester bond in ADPr-Ub. Then the sample was processed in two different mass spectrometry, ETD and HCD. In ETD, ADPr-Ub is not fragmented, integrated ion peaks are detected, to probe the exact modification site on peptide fragments. In HCD, a more intensive condition, the ADPr-Ub is fragmented to produce characteristic peaks account for ADPr-Ub modification such as ADP-GG and AMP-GG. PARP1 and H3, H2B were found substrates for ADPr-Ub modification in cell.
In addition, the article engineered a ZUD-HRP conjugate to visualize cellular ADPr-Ub modification in immunoblotting. They succeeded in visualize H3 ADPr-Ub and PARP1 ADPr-Ub modification. In summary, the article established a robust strategy to verify ADPr-Ub modification sites in cell. However, the biological importance of ADPr-Ub is still under explored. Hitherto, little is known about how ADPr-Ub modification work in cells.
doi
10.1038/s41589-025-01974-5