Article Summary 6/21/25
Article title
BRAF oncogenic mutants evade autoinhibition through a common mechanism
Journal
Science
Tags
BRAF kinase; RAS-ERK pathway; Structural biology; Oncogenic mutation
Introduction
BRAF kinase, which is involved in RAS-ERK signaling pathway, is closely related to cancers. Oncogenic mutations of BRAF, such as BRAF V600E, which is widely implied in cancers, lead to uncontrolled activation of BRAF kinase activity, thus promoting tumor generation.
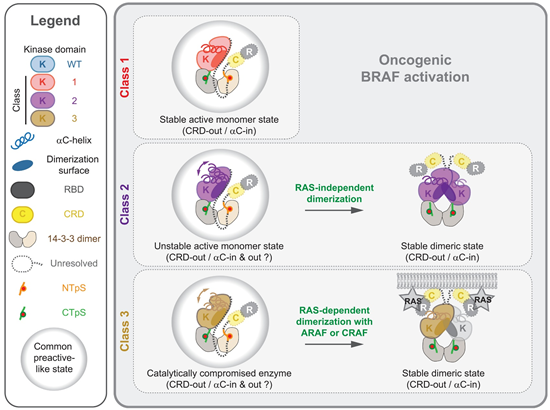
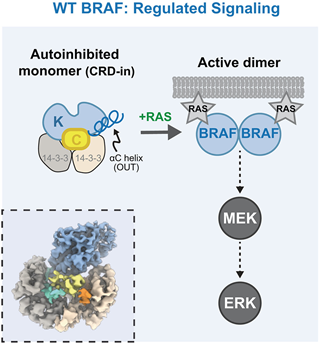
BRAF activity regulation involves auto-inhibited monomer state in complex with 2 copies of 14-3-3 protein and activated dimer state in complex with RAS. Structure of these states have been reported. However, how BRAF oncogenic mutations affect the regulation of BRAF activation remains elusive.
This work
The cryo-EM structure of the most abundant BRAF oncogenic mutation, V600E in complex with 14-3-3 was resolved. Different from wildtype BRAF, the CRD domain in V600E mutant is invisible under observation, lacking CRD-KD intramolecular interaction in wildtype BRAF, indicating a CRD-out conformation for V600E. Next, biochemical characterization is performed to verify the CRD-KD interaction and its impact on downstream RAS-ERK pathway, which is indexed by anti-pERK immunoblot.
Therefore, they seek to reverse the CRD-out conformation through BRAF-targeted inhibitors. PLX8394, the paradox breaker inhibitor of BRAF, is selected to determine its complex with BRAF and 14-3-3. With presence of PLX8394, BRAF retained CTD-in conformation, revealing that PLX8394 and such inhibitors regulates BRAF through controlling its CRD-in and CRD-out conformation. To confirm the universality of CRD-in/out regulation mechanism, cryo-EM structures of BRAF Class II/III (BRAF V600E: Class I) bound to 14-3-3 were resolved. Both Class II and Class III mutants showed CRD-out conformation, implying common feature of CRD-out conformation in BRAF mutants.