Article summary 04-26-25
Article title
Covalent adduct Grob fragmentation underlies LSD1 demethylase-specific inhibitor mechanism of action and resistance
Journal
Nature Communications
Tags
Inhibitor mode of action; LSD1 inhibitor; covalent drug
Introduction
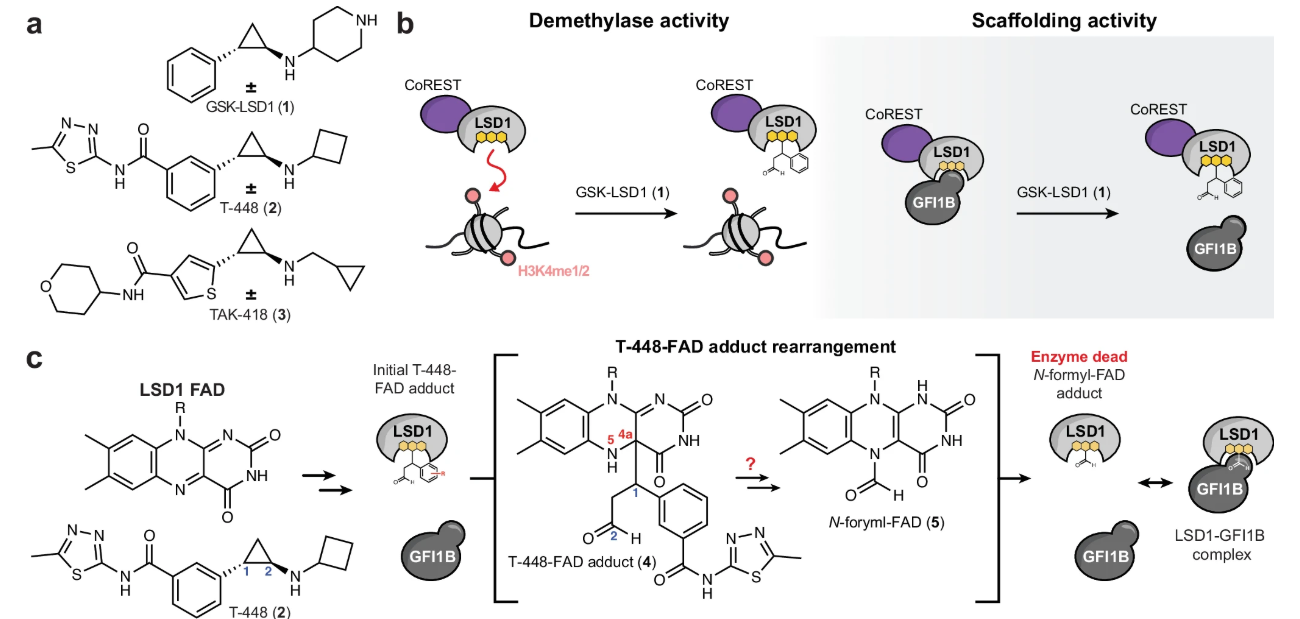
Targeting epigenetic-relevant regulators is attractive therapeutic approach. Inhibitor targeting chromatin demethylase LSD1 has been explored, one family is called TCP, in which the inhibitors capture LSD1 active site by forming covalent adduct with LSD1 cofactor FAD. The covalent adduct not only inhibits demethylation activity but also hampers LSD1 interaction with transcription factor GFI1B to treat cancers such as leukemia. However, some TCP inhibitors, such as T448, undergo rearrangement following adduct formation and lose the ability to inhibit GFI1B binding. The mechanism of the rearrangement and how its rearrangement is regulated remains unexplored.
This work
Using chemical-based approach, this work first demonstrated the rearrangement is a Grob fragmentation, yielding styrene as eliminated product. The impact of substitution groups towards fragmentation was screened. Then, to structurally characterize the fragmentation, crystal structure of LSD1-inhibitor is analyzed. Pre-fragmentation and post-fragmentation structures were determined. In addition, by CRISPR-suppressor screening, LSD1 loop deletion could bind GFI1B in the presence of inhibitor AW4 (AW4 will not rearrange when binding to WT LSD1). Advanced structural characterization revealed LSD1 loop interacts with αD-helix of LSD1 to promote fragmentation.
Collectively, this work clarify the mechanism of Grob fragmentation-mediated LSD1 inhibitor function divergence. This work provides insights towards the function of LSD1 inhibitors and potential LSD1 drug-resistant mechanism.
doi
doi.org/10.1038/s41467-025-57477-3