Article Summary 9/14/24
Article title
Cancer-associated DNA hypermethylation of Polycomb targets requires DNMT3A dual recognition of histone H2AK119 ubiquitination and the nucleosome acidic patch
Journal
Science Advances
Tags
DNA methylation; Ubiquitinated nucleosome complex; Genetics
Introduction
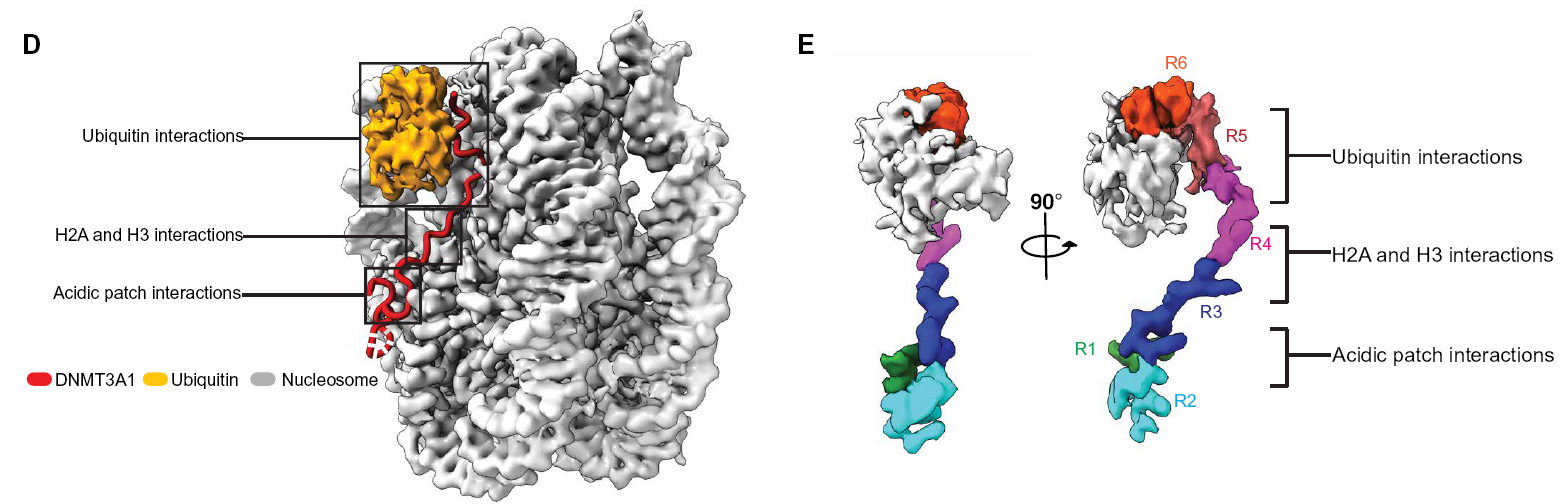
DNA methylation is vital epigenetic mark, dysregulation of DNA methylation causes disease. De novo chromatin DNA methylation is mediated by DNMT3A. An isoform DNMT3A1, mainly locates on heterochromatin. DNMT3A1 utilizes its PTM reader domains, such as UDR to H2AK119Ub, to correctly target specific chromatin foci, but in diseases such as neurocrine cancers, pathogenic DNMT3A1 mutation results in redistribution of DNMT3A1 on chromatin, making it enriched in polycomb promoters, in which H2AK119Ub is abundant. However, the mechanism of DNMT3A1 redistribution on chromatin polycomb promoter remain elusive.
This work
Determine the cryo-EM structure of DNMT3A1 UDR domain bound to H2AK119Ub NCP (seemingly like SSX1-H2A119Ub structure). Based on the structure, a vital mutant that eliminates DNMT3A1-H2AK119Ub NCP was discovered. Using this mutant, it is found that the DNMT3A1-H2AK119Ub NCP interaction regulates the hypermethylation on redistributed DNMT3A1-enriched chromatin polycomb promoter.
Supplemented the structure with much sequencing data, such as RRBS to detect methylation landscape, as well as RNA-seq to probe transcripts change followed by DNMT3A1 mutation. As well, cancer model mice were adapted to illustrate the importance of UDR-NCP interaction in cancer progression.
doi
10.1126/sciadv.adp0975